Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:6 Percentile:58.16(Multidisciplinary Sciences)no abstracts in English
 -dimethylformamide
-dimethylformamideKim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Ikeda, Yasuhisa*
Journal of Alloys and Compounds, 408-412, p.1291 - 1295, 2006/02
Times Cited Count:10 Percentile:54.65(Chemistry, Physical)The electrochemical reactions of UO (
( -diketonato)
-diketonato) DMF, UO
DMF, UO (trop)
(trop) DMF and UO
DMF and UO (sap)(DMF)
(sap)(DMF) , (DMF = N,N-dimethylformamide,
, (DMF = N,N-dimethylformamide,  -diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E
-diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E ,
,  . ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO
. ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO (ttfa)
(ttfa) DMF, -1.18 V for UO
DMF, -1.18 V for UO (btfa)
(btfa) DMF, -1.46 V for UO
DMF, -1.46 V for UO (dbm)
(dbm) DMF, -1.46 V for UO
DMF, -1.46 V for UO (trop)
(trop) DMF, and -1.59 V for UO
DMF, and -1.59 V for UO (sap)(DMF)
(sap)(DMF) .
.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji
Radiochimica Acta, 93(12), p.767 - 770, 2005/12
Times Cited Count:10 Percentile:56.74(Chemistry, Inorganic & Nuclear)Electrochemical and spectroelectrochemical has been used to investigate the behaviour of Neptunium (VI) in concentration 1-8 M HNO solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E
solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E ) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO
) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO
solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO
solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO solution.
solution.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji
JAERI-Conf 2005-007, p.341 - 344, 2005/08
Electrochemistry has been used to investigate the behavior of plutonium(IV) in 1-7 M HNO solutions. These Pu(IV) complexes were found to be reduced quasi-reversibly to Pu(III) species. The formal redox potentials (E
solutions. These Pu(IV) complexes were found to be reduced quasi-reversibly to Pu(III) species. The formal redox potentials (E ) for Pu(IV)/Pu(III) couples were determined to be +0.721, +0.712, +0.706, +0.705, +0.704, 0.694, and +0.696 V (vs. Ag/AgCl(SSE)) for Pu(IV) complexes in 1, 2, 3, 4, 5, 6, 7 M HNO
) for Pu(IV)/Pu(III) couples were determined to be +0.721, +0.712, +0.706, +0.705, +0.704, 0.694, and +0.696 V (vs. Ag/AgCl(SSE)) for Pu(IV) complexes in 1, 2, 3, 4, 5, 6, 7 M HNO solutions, respectively. These results indicate that the reduction product of Pu(IV) is Pu(III), which is considerably stable in HNO
solutions, respectively. These results indicate that the reduction product of Pu(IV) is Pu(III), which is considerably stable in HNO solution.
solution.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Radiochimica Acta, 93(2), p.75 - 81, 2005/02
Times Cited Count:9 Percentile:53.19(Chemistry, Inorganic & Nuclear)no abstracts in English
 resolution
resolutionChatake, Toshiyuki*; Kurihara, Kazuo; Tanaka, Ichiro*; Tsyba, I.*; Bau, R.*; Jenney, F. E. Jr.*; Adams, M. W. W.*; Niimura, Nobuo
Acta Crystallographica Section D, 60(8), p.1364 - 1373, 2004/08
Times Cited Count:34 Percentile:88.89(Biochemical Research Methods)A neutron diffraction study has been carried out at 1.6  resolution on a mutant rubredoxin from
resolution on a mutant rubredoxin from 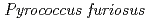 using the BIX-3 single-crystal diffractometer at the JRR-3 reactor of JAERI. In order to study the unusual thermostability of rubredoxin from
using the BIX-3 single-crystal diffractometer at the JRR-3 reactor of JAERI. In order to study the unusual thermostability of rubredoxin from 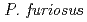 , the hydrogen-bonding patterns were compared between the native and a 'triple-mutant' variant where three residues were changed so that they are identical to those in a mesophilic rubredoxin. In the present study, some minor changes were found between the wild-type and mutant proteins in the hydrogen-bonding patterns of the Trp3/Tyr3 region. The H/D-exchange ratios in the protein were also studied. The results suggest that the backbone amide bonds near the four Cys residues of the FeS
, the hydrogen-bonding patterns were compared between the native and a 'triple-mutant' variant where three residues were changed so that they are identical to those in a mesophilic rubredoxin. In the present study, some minor changes were found between the wild-type and mutant proteins in the hydrogen-bonding patterns of the Trp3/Tyr3 region. The H/D-exchange ratios in the protein were also studied. The results suggest that the backbone amide bonds near the four Cys residues of the FeS redox center are most resistant to H/D exchange. In addition, the 1.6
redox center are most resistant to H/D exchange. In addition, the 1.6  resolution of the present neutron structure determination has revealed a more detailed picture than previously available of some portions of the water structure, including ordered and disordered O-D bonds.
resolution of the present neutron structure determination has revealed a more detailed picture than previously available of some portions of the water structure, including ordered and disordered O-D bonds.
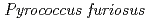 by BIX-3, a single-crystal diffractometer for biomacromolecules
by BIX-3, a single-crystal diffractometer for biomacromoleculesKurihara, Kazuo; Tanaka, Ichiro*; Chatake, Toshiyuki*; Adams, M. W. W.*; Jenney, F. E. Jr.*; Moiseeva, N.*; Bau, R.*; Niimura, Nobuo
Proceedings of the National Academy of Sciences of the United States of America, 101(31), p.11215 - 11220, 2004/08
Times Cited Count:48 Percentile:61.13(Multidisciplinary Sciences)The structure of a rubredoxin (Rd) from  , an organism that grows optimally at 100
, an organism that grows optimally at 100  C, was determined using the neutron single-crystal diffractometer for biological macromolecules (BIX-3) at the JRR-3 reactor of JAERI. Data were collected at room temperature up to a resolution of 1.5
C, was determined using the neutron single-crystal diffractometer for biological macromolecules (BIX-3) at the JRR-3 reactor of JAERI. Data were collected at room temperature up to a resolution of 1.5  , and the completeness of the data set was 81.9 %. The model contains 306 H atoms and 50 D atoms. A total of 37 hydration water molecules were identified. The model has been refined to final agreement factors of
, and the completeness of the data set was 81.9 %. The model contains 306 H atoms and 50 D atoms. A total of 37 hydration water molecules were identified. The model has been refined to final agreement factors of  = 18.6 % and
= 18.6 % and 
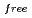 = 21.7 %. Several orientations of the O-D bonds of side chains, whose assignments from X-ray data were previously ambiguous, were clearly visible in the neutron structure. While most backbone N-H bonds had undergone some degree of H/D exchange throughout the molecule, five H atom positions still had distinctly negative (H) peaks. The neutron Fourier maps clearly showed the details of an extensive set of H bonds involving the ND
= 21.7 %. Several orientations of the O-D bonds of side chains, whose assignments from X-ray data were previously ambiguous, were clearly visible in the neutron structure. While most backbone N-H bonds had undergone some degree of H/D exchange throughout the molecule, five H atom positions still had distinctly negative (H) peaks. The neutron Fourier maps clearly showed the details of an extensive set of H bonds involving the ND
 terminus that may contribute to the unusual thermostability of this molecule.
terminus that may contribute to the unusual thermostability of this molecule.
 -ray
-rayOkagawa, Seigo; Nagai, Hitoshi*; Abe, Hitoshi*; Tashiro, Shinsuke
JAERI-Tech 2003-068, 17 Pages, 2003/08
To study the release mechanism of iodine from the dissolver solution in the nuclear fuel reprocessing plants to the atmosphere at criticality accidents, the characteristics of redox of iodine species in various solutions must be examined. In the present work, the effect of  -ray irradiation on the redox reaction was examined in the nitric acid solution of 1M and 3M. Without irradiation, most of iodine in 1M nitric acid solutions were exist in the form of I
-ray irradiation on the redox reaction was examined in the nitric acid solution of 1M and 3M. Without irradiation, most of iodine in 1M nitric acid solutions were exist in the form of I
 . In 3M nitric acid solutions, iodine was oxidized to I
. In 3M nitric acid solutions, iodine was oxidized to I . When the solutions were irradiated by
. When the solutions were irradiated by  -ray with exposure of more than 4C/kg, I
-ray with exposure of more than 4C/kg, I
 was disappeared regardless of nitric acid concentration. At exposure of 120C/kg, iodine was oxidized to IO
was disappeared regardless of nitric acid concentration. At exposure of 120C/kg, iodine was oxidized to IO
 . At exposure of 4800C/kg, iodine was exist in the form of IO
. At exposure of 4800C/kg, iodine was exist in the form of IO
 in 1M nitric acid solution. On the other hand, iodine in 3M nitric acid solution was reduced to I
in 1M nitric acid solution. On the other hand, iodine in 3M nitric acid solution was reduced to I at the same exposure. In the irradiated solution, nitrous acid was found, which would be produced from nitric acid by
at the same exposure. In the irradiated solution, nitrous acid was found, which would be produced from nitric acid by  -ray irradiation.
-ray irradiation.
Kato, Chiaki
JAERI-Research 2003-013, 143 Pages, 2003/08
This study is investigation about stress corrosion cracking (SCC) of zirconium in nuclear fuel reprocessing. Chapter 1 is described background. Chapter 2 is explained experimental apparates. Chapter 3 is described the increased oxidization potential on the heat-transfer surface and suggested the initiation of SCC on a boiling heat-transfer surface. Chapter 4 is described that the SCC susceptibility increased with increasing nitric acid concentration and solution temperature on notched specimen by SSRT. In addition, the SCC susceptibility effected by the crystal anisotropy by the hot rolling direction and increased on a parallel face to the rolling direction. Chapter 5 is described that the SCC susceptibility increased in HAZ/base metal boundary in order to the preferential orientation of cleavage plane (0002). Chapter 6 is described that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition on the surface and the removal of the decomposition product from solution by boiling bubbles.
Matsumoto, Shiro*; Uchiyama, Gunzo; Ozawa, Masaki*; Kobayashi, Y.*; Shirato, K.*
Radiochemistry, 45(3), p.219 - 224, 2003/05
no abstracts in English
Motooka, Takafumi; Kiuchi, Kiyoshi
Journal of Nuclear Science and Technology, 39(Suppl.3), p.367 - 370, 2002/11
A corrosion of a stainless steel in nitric acid solution containing Np has been studied. Using SUS304L stainless steel, corrosion tests in boiling neptunium nitrate solution were conducted under immersion and heat-transfer condition. By the weight loss measurement of stainless steel and the quantitative analysis of metallic ions dissolved in solution, the corrosion rates were obtained. The surface morphology was observed by scanning electron microscopy. The acceleration mechanism was investigated by electrochemistry and spectrophotometry. The corrosion rate was accelerated by addition of neptunium in nitric acid. Preferential intergranular corrosion was observed. The corrosion was enhanced under heat-transfer condition compared to immersion condition. In polarization measurements, the cathodic over-voltage was decreased; the cathodic current was increased by addition of Np. Spectrophotometric measurements showed that Np(V) oxidized to Np(VI) in boiling nitric acid. The corrosion acceleration mechanism in nitric acid solution containing Np suggested the re-oxidation of Np(V).
Nagao, Seiya; Yanase, Nobuyuki; Yamamoto, Masayoshi*; Kofuji, H.*; Sorin, Yoshiki*; Amano, Hikaru
Journal of Radioanalytical and Nuclear Chemistry, 252(2), p.225 - 232, 2002/05
Times Cited Count:9 Percentile:51.46(Chemistry, Analytical)no abstracts in English
Kurihara, Kazuo; Tanaka, Ichiro; Adams, M. W. W.*; Jenney, F. E. Jr.*; Moiseeva, N.*; Bau, R.*; Niimura, Nobuo
Journal of the Physical Society of Japan, Vol.70, Supplement A, p.400 - 402, 2001/05
With the new single-crystal diffractometer BIX-3 at the JRR-3M reactor of JAERI, a single-crystal neutron diffraction analysis of the structure of the small protein rubredoxin from the hyperthermophile Pyrococcus furiosus is currently under way. Data were collected at room temperature up to a resolution of 1.5  intervals in
intervals in  and exposure times ranging from 60 to 77 minutes per frame. The completeness factor of the 1.5-
and exposure times ranging from 60 to 77 minutes per frame. The completeness factor of the 1.5- . Included in the refinement are 301 hydrogen atoms and 40 deuterium atoms, and 29 water molecules were also identified. In the present model, the current value for R and R
. Included in the refinement are 301 hydrogen atoms and 40 deuterium atoms, and 29 water molecules were also identified. In the present model, the current value for R and R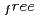 are 24.0
are 24.0 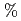 and 26.3
and 26.3 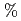 , respectively.
, respectively.
Uchiyama, Gunzo; Fujine, Sachio; Yoshida, Zenko; Maeda, Mitsuru; *
JAERI-Research 98-005, 20 Pages, 1998/02
no abstracts in English
*;
Nuclear Technology, 97, p.323 - 335, 1992/03
Times Cited Count:3 Percentile:35.32(Nuclear Science & Technology)no abstracts in English
Tachimori, Shoichi
Journal of Nuclear Science and Technology, 28(3), p.218 - 227, 1991/03
no abstracts in English
J.Ahn*;
Proc. of the 3rd Int. Symp. on Advanced Nuclear Energy Research; Global Environment and Nuclear Energy, 10 Pages, 1991/00
no abstracts in English
Tachimori, Shoichi
JAERI-M 90-018, 86 Pages, 1990/02
no abstracts in English
Suwa, Takeshi; ; *
Dekomisshoningu Giho, (2), p.29 - 40, 1990/00
no abstracts in English
Hocus Cruise Report in Environmental Studies Series, p.145 - 150, 1988/00
no abstracts in English